Imagine a group of marathon runners rushing past a novice photographer. The man was able to capture a few moments through his lenses. But to his disappointment, nearly all his pictures were bleary and out of focus. Quickly realizing his grave mistake, he checked the shutter speed...It was accidentally lowered, to his surprise. This silly error cost him a few good pictures.
So now we realize the importance of Shutter Speed. It is the length of time for which the camera sensor is exposed to light, or in other words - the time the camera spends to click one picture. It affects the quality of the image tremendously. If the shutter speed is lowered to maybe 1/50, fast-moving objects like the hummingbird's wings will rapidly move numerous times by the time the shutter closes - resulting in an out-of-focus picture as shown below.
Now for a moment let's divert our attention to the study of microscopic entities. To study any object seemingly invisible to our naked eye we 'shower' the object under study with particles, which on interaction with the object gives us an idea about its structure. The tinier the particles that are 'showered' - the more accurate visualization we have about the object's structural character. This concept is illustrated using a lucid, clear diagram from the famous book, 'The Elegant Universe'(Brian Greene).
'The Elegant Universe' by Brian Greene
Using this analogy we can make out how a microscope 'conjures' accurate images of the microscopic realm. But when we delve further down the scale - we realize that a light microscope has to be replaced by an Electron Microscope - which uses magnets to direct electrons toward the specimen, resulting in images with unprecedented magnification and accuracy.
The reason for the increased magnification of the Electron microscope is simple - the ingenious idea to use electrons instead of visible light. Since the wavelength of an electron can be up to 100,000 smaller than that of visible light. By decreasing the wavelength we are essentially increasing our 'shutter speed' - hence allowing us to study the structure and movements related to microscopic entities to the fullest.
What if we now want to study the movements of an electron? We are going to face the following issues:
- The electron is very small. Its diameter is in the order of 10⁻¹⁴. Therefore, its movements are impossible to detect and study with pinpoint precision.
- Rearrangements and movements of electrons in atoms and molecules occur in a period of magnitude 1 x 10⁻¹⁸ s or in Attoseconds. Hence to properly study electrons we need wave pulses with time periods in the order of attoseconds to actually 'see' the movements of electrons. BUT we did not possess any method to generate these attosecond pulses. Therefore, studying the movements of electrons with such desired precision was a castle in the air.
The Torchbearers and their Research:
Physically it is impossible to turn a button on and off to produce attosecond pulses, so indirect methods were the need of the hour. In the early 1990s Anne L'Huillier found out that when laser light with long wavelengths is incident upon some rare gases, waves of higher frequencies are being emitted from the gaseous atoms. This method was later used to produce attosecond pulses of light.
When several waves with evenly spaced frequencies 'combine' or interfere the resultant waves will be similar to 'pulses' as shown below.
So the Nobel Prize-winning experimentalists used this 'age-old' known technique to produce pulses that are in the order of 10⁻¹⁸ s or attoseconds. These pulses came to be known as 'overtones'.
The Underlying Reason For the Emission of Overtones:
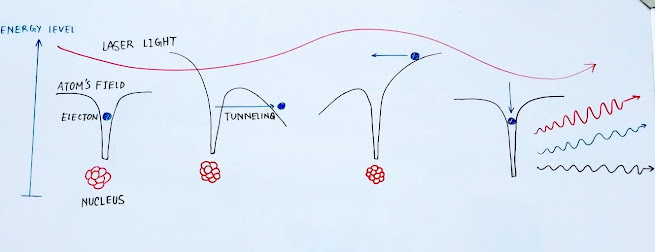
The electron is bound by the coulombic force of attraction of the nucleus. As illustrated in the diagram below, the electron is within the influence of the strong field of the atom. The laser itself is an electromagnetic wave, hence on coming close to the atom the laser's electric field disturbs the potential of the electron. Therefore, the influence of the atoms's field is minimized and the electron may even be torn apart. Or, in the words of quantum mechanics the electron 'tunnels' out of the atom's field since the barrier is weakened by the laser's field(electric).
But when the half cycle of the electric field of the laser is over, the electron falls back into the influence of the atom's strong electric field. Since the electron was ripped out of the atom and instantaneously made to return - it gained some energy. This extra energy is given out in the form of overtones. The overtones released from specific rare gases interfere to produce attosecond pulses.
Applications Of Attosecond Physics:
- Electron dynamics: This is the most prominent application of attosecond physics. By generating attosecond pulses of light we can probe into sub-atomic events that were earlier thought to be instantaneous. For example, according to the photoelectric effect, if a photon has sufficient energy it may cause electrons to be ejected from a material after colliding with it. Ferenc Krausz used attosecond pulses to determine the delay in the ejection of electrons from the atoms of the material. It was found that there is a delay of 21 Attoseconds between the ejection of the electron in 2s orbital and 2p orbital. Such studies can be made possible only with Attoseconds physics.
- Ferromagnetic materials: Attosecond pulses can be used to align the spin of ferromagnetic particles in a very short period. Therefore reducing the time taken to induce magnetic properties in such materials.
- Semiconductors: We use semiconductors for myriad purposes in our day-to-day life. These essentially consist of electrons jumping on and off different conduction bands. By using attosecond physics we can track and analyze the movements of electrons in these semiconductors.









2 Comments
An informative one . Well done 👍
ReplyDeleteThank you!! 😄
ReplyDelete